X-Ray Data Booklet
Section 3.1 SCATTERING of X-RAYS from ELECTRONS and ATOMS
A. COHERENT, RAYLEIGH, OR ELASTIC SCATTERING
Scattering from single electrons (Thomson scattering) has a total cross section
Scattering from single electrons (Thomson scattering) has a total cross section
(1)
where
![]() is
the classical radius of the electron,
is
the classical radius of the electron, ![]()
![]() meter.
The angular distribution for unpolarized incident radiation is proportional
to
meter.
The angular distribution for unpolarized incident radiation is proportional
to ![]() , where q
is the scattering angle. For polarized incident radiation, the cross section
vanishes at 90° in the plane of polarization.
, where q
is the scattering angle. For polarized incident radiation, the cross section
vanishes at 90° in the plane of polarization.
Scattering from atoms involves the cooperative effect of all the electrons, and the cross section becomes
(2)
where
![]() is
the (complex) atomic scattering factor, tabulated in Section 2.7 of this booklet.
Up to about 2 keV, the scattering factor is approximately independent of scattering
angle, with a real part that represents the effective number of electrons
that participate in the scattering. At higher energies, the scattering factor
falls off rapidly with scattering angle. For details see Ref. 1.
is
the (complex) atomic scattering factor, tabulated in Section 2.7 of this booklet.
Up to about 2 keV, the scattering factor is approximately independent of scattering
angle, with a real part that represents the effective number of electrons
that participate in the scattering. At higher energies, the scattering factor
falls off rapidly with scattering angle. For details see Ref. 1.
In relativistic quantum mechanics, the scattering of x-rays by a free electron is given by the Klein-Nishina formula. If we assume unpolarized x-rays and unaligned electrons, this formula can be approximated as follows for x-ray energies below 100 keV:
(3)
where
![]() , the photon energy
measured in units of the electron rest energy. The total cross section is
approximately
, the photon energy
measured in units of the electron rest energy. The total cross section is
approximately
(4)
Note
that for very low energies ![]() , we recover the
Thomson cross section. The real difference comes when we deal with atoms.
In that case, if the scattering leaves the atom in the ground state, we deal
with coherent scattering (see above), whereas if the electron is ejected from
the atoms, the scattering is (incoherent) Compton scattering. At high energies,
the total Compton cross section approaches
, we recover the
Thomson cross section. The real difference comes when we deal with atoms.
In that case, if the scattering leaves the atom in the ground state, we deal
with coherent scattering (see above), whereas if the electron is ejected from
the atoms, the scattering is (incoherent) Compton scattering. At high energies,
the total Compton cross section approaches ![]() . At low energies
and small scattering angles, however, binding effects are very important,
the Compton cross section is significantly reduced, and coherent scattering
dominates (see Figs. 3-1 and 3-2). For details see Refs. 1 and 2.
. At low energies
and small scattering angles, however, binding effects are very important,
the Compton cross section is significantly reduced, and coherent scattering
dominates (see Figs. 3-1 and 3-2). For details see Refs. 1 and 2.
The scattered x-ray suffers an energy loss, which (ignoring binding effects) is given by
(5)
or, in terms of the wavelength shift,
(6)
where
![]() .
The kinetic energy of the recoil electron is just the energy lost by
the photon in this approximation:
.
The kinetic energy of the recoil electron is just the energy lost by
the photon in this approximation:
(7)
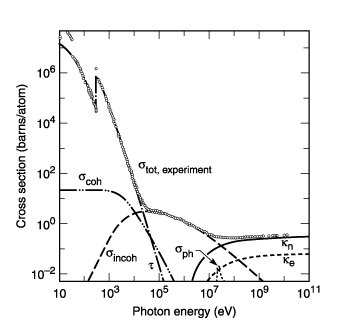
Fig. 3-1. Total photon cross section ![]() in
carbon, as a function of energy, showing the contributions of different processes:
t, atomic photo-effect (electron ejection, photon
absorption);
in
carbon, as a function of energy, showing the contributions of different processes:
t, atomic photo-effect (electron ejection, photon
absorption); ![]() , coherent scattering
(Rayleigh scattering—atom neither ionized nor excited);
, coherent scattering
(Rayleigh scattering—atom neither ionized nor excited); ![]() , incoherent scattering
(Comp- ton scattering off an electron);
, incoherent scattering
(Comp- ton scattering off an electron); ![]() , pair production,
nuclear field;
, pair production,
nuclear field; ![]() , pair production,
electron field;
, pair production,
electron field; ![]() , photonuclear absorption
(nuclear absorption, usually followed by emission of a neutron or other particle).
(From Ref. 3; figure courtesy of J. H. Hubbell.)
, photonuclear absorption
(nuclear absorption, usually followed by emission of a neutron or other particle).
(From Ref. 3; figure courtesy of J. H. Hubbell.)
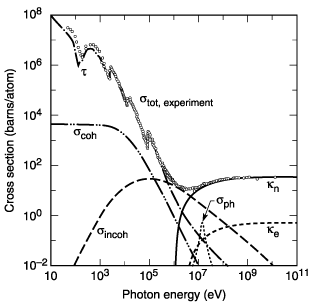
Fig. 3-2. Total photon cross section ![]() in
lead, as a function of energy. See Fig. 3-1. (From Ref. 3; figure courtesy
of J. H. Hubbell.)
in
lead, as a function of energy. See Fig. 3-1. (From Ref. 3; figure courtesy
of J. H. Hubbell.)
REFERENCES
1. J. H. Hubbell, W. J. Veigele, E. A. Briggs, R. T. Brown, D. T. Cromer, and R. J. Howerton, “Atomic Form Factors, Incoherent Scattering Functions, and Photon Scattering Cross Sections,” J. Phys. Chem. Ref. Data 4, 471 (1975).
2. R. D. Evans, The Atomic Nucleus (Kreiger, Malabar, FL, 1982); R. D. Evans, “The Compton Effect,” in S. Flugge, Ed., Handbuch der Physik, vol. 34 (Springer-Verlag, Berlin, 1958), p. 218; W. J. Veigele, P. T. Tracy, and E. M. Henry, “Compton Effect and Electron Binding,” Am. J. Phys. 34, 1116 (1966).
3. J. H. Hubbell, H. A. Gimm, I. ![]() , “Pair, Triplet,
and Total Atomic Cross Sections (and Mass Attenuation Coefficients) for 1
MeV–100 GeV Photons in Elements Z = 1 to 100,” J.
Phys. Chem. Ref. Data 9, 1023 (1980).
, “Pair, Triplet,
and Total Atomic Cross Sections (and Mass Attenuation Coefficients) for 1
MeV–100 GeV Photons in Elements Z = 1 to 100,” J.
Phys. Chem. Ref. Data 9, 1023 (1980).